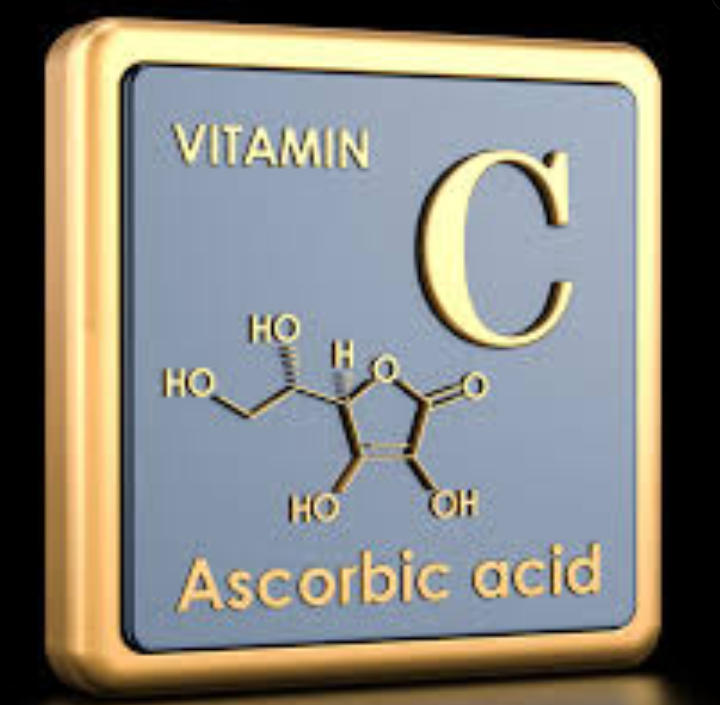
Ah, ascorbic acid — better known as vitamin C — is one of those rare molecules that stands at the crossroads of nutrition, preservation, and chemistry’s quiet poetry.
Let’s explore what it is, what it does, and why it remains indispensable to both the human body and the global food industry.
🧪 What Exactly Is Ascorbic Acid?
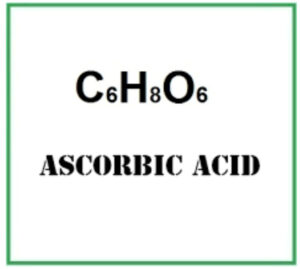
Chemically speaking, ascorbic acid (C₆H₈O₆) is a water-soluble organic acid derived from glucose.
It’s a powerful antioxidant — a molecular guardian that willingly gives up electrons to neutralize free radicals, preventing oxidative damage in both living tissue and food systems.
It’s naturally found in:
Citrus fruits (orange, lemon, lime)
Kiwi, strawberry, guava
Tomatoes, peppers, broccoli
Acerola and camu-camu (extremely rich sources)
Industrial production typically follows two paths:
Biotechnological (Reichstein or two-step fermentation) from glucose using microbial oxidation and hydrogenation.
Fully fermentation-based modern processes, where genetically engineered microorganisms convert glucose directly into ascorbic acid — cleaner and greener.

💊 Why Is It Essential for Human Health?
Humans, unlike many animals, cannot synthesize vitamin C — we lost that metabolic ability long ago. Hence, it must come from our diet.
Its biological roles are both vast and vital:
| Function | Description |
|---|---|
| Collagen synthesis | Acts as a cofactor for enzymes that stabilize collagen — the protein scaffolding of skin, cartilage, blood vessels, and bone. |
| Antioxidant defense | Neutralizes reactive oxygen species (ROS), regenerates vitamin E, and protects DNA, lipids, and proteins from oxidative harm. |
| Immune support | Enhances white blood cell activity and aids in infection resistance. |
| Iron absorption | Converts ferric (Fe³⁺) to ferrous (Fe²⁺) iron, improving uptake from plant sources. |
| Wound healing | Essential for new tissue formation and scar integrity. |
| Neurotransmitter synthesis | Participates in converting dopamine to norepinephrine. |
Deficiency leads to scurvy — fatigue, gum bleeding, joint pain, and fragile skin — the very disease that once haunted sailors.
The recommended daily intake:
~75 mg (women), ~90 mg (men); higher in smokers or stress-prone individuals.
🧁 Why Is It So Valuable in the Food Industry?
Ascorbic acid’s antioxidant power makes it a natural protector of color, flavor, and freshness.
In Foods and Beverages
| Application | Function |
|---|---|
| Beverages & juices | Prevents oxidation, preserves flavor and vitamin content. |
| Bakery products | Strengthens gluten network (acts as a dough improver/oxidizer), improves loaf volume and crumb texture. |
| Processed meats | Reduces nitrite → nitric oxide, stabilizing cured color; slows rancidity. |
| Fruits & vegetables | Prevents enzymatic browning (especially in fresh-cut produce). |
| Oils & fats | Delays oxidation and rancid odor development. |
 It’s listed on ingredient labels as:
It’s listed on ingredient labels as:
Ascorbic acid (E300)
and sometimes as its salts:
Sodium ascorbate (E301), Calcium ascorbate (E302).
🌍 The Dual Life of Ascorbic Acid — in Health and Commerce
In human biology, it saves cells from rusting.
In food systems, it saves flavor from fading.
It’s both a nutrient and a stabilizer — a bridge between life’s chemistry and the chemistry of preservation.
Modern consumers may fear “additives,” but here is an additive that is also a vitamin, a symbol of how chemistry and nature collaborate rather than compete.
✨ Summary
| Aspect | Role |
|---|---|
| Nature | Vitamin C, antioxidant compound |
| Main sources | Citrus, berries, vegetables |
| Human importance | Collagen synthesis, immune defense, antioxidant |
| Food use | Preservative, dough improver, color stabilizer |
| Label name | Ascorbic acid (E300) |
| Deficiency symptom | Scurvy |